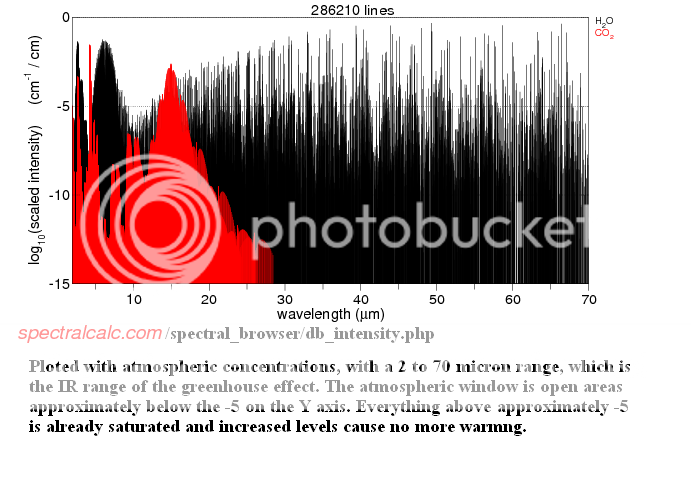
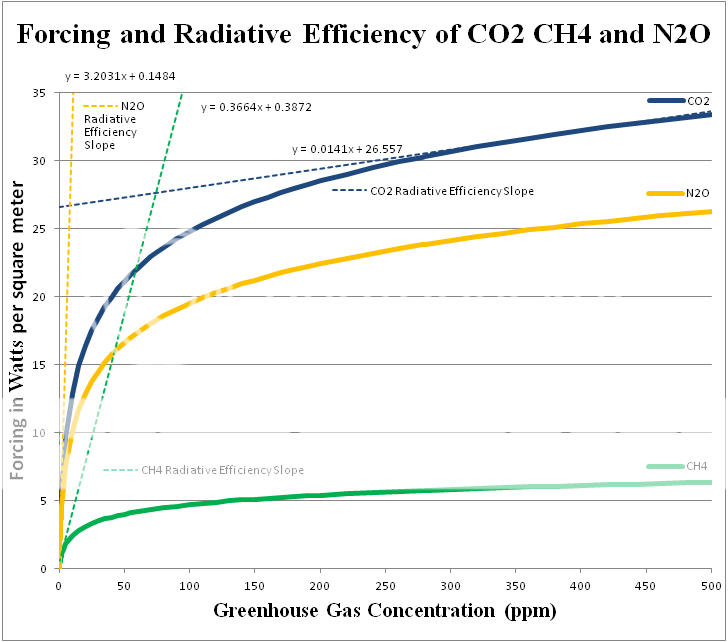
There are a couple of important differences between H2O and less abundant greenhouse gases like CO2, CH4 and N2O which Longview and Lord of Planar apparently either don't know, or have chosen not to mention in their would-be condescending diatribes.
The
atmospheric limits of water vapour is probably most relevant to your question: Most H2O is in the form of liquid water at atmospheric temperature and pressure (if not solid ice!), every schoolkid knows that. The global average surface air temperature is about 15 degrees Celsius, and at that temperature the dew point of water vapour (100% humidity) is only about 11 grams per kilogram of air (1.1% by mass, which I believe is around 1.66% by volume). The global average concentration of atmospheric water vapour is around 2% by volume if memory serves, as L and L's figures also suggest, presumably because temperature and pressure vary around the world and with altitude. But in most places there's likely to be little if any room for increase in atmospheric water vapour. Increases in global temperatures could raise that threshold a little, but water vapour alone can't accomplish that.
https://en.wikipedia.org/wiki/Relative_humidity#Other_important_facts
https://en.wikipedia.org/wiki/Water_vapor#In_Earth.27s_atmosphere
Secondly, the
uneven distribution of water vapour throughout the atmosphere - both vertically and horizontally. Total column water vapour content is in the order of 6-20 times greater around the tropics than at the Arctic or Antarctic circles. Over 99% of water vapour is contained in the troposphere. By contrast, concentration changes of gases which are not so dramatically affected by small temperature and pressure changes (like CO2, CH4 and N2O) last longer so that they have time to become distributed more evenly throughout the troposphere; hence they're called well-mixed greenhouse gases. CO2 also has a uniform distribution ratio vertically, at least up to 80km or so, well above the stratosphere. The CH4 and N2O ratios decrease somewhat in the stratosphere, but not nearly as much as water vapour does: There's nearly 10,000 times as much H2O at the surface as there is CH4, but in the lower stratosphere there's less than twice as much!
https://en.wikipedia.org/wiki/Water_vapor#Radar_and_satellite_imaging
http://ruc.noaa.gov/AMB_Publication...osition and Vertical Structure_eae319MS-1.pdf
It's worth noting that this is extremely old information: The first was the reason why, back in the 19th century, Svante Arrhenius concluded that CO2 was more likely as a candidate for helping to regulate the Pleistocene climate's glacial/interglacial cycles than water vapour; because it's got a much better chance to maintain persistent change over time. The "saturated absorption bands" argument which L and L are making was a legitimate objection to anthropogenic global warming... about 70 years ago. In fact the necessarily dry conditions in the cold upper atmosphere were also known to Arrhenius and noted in response to his critics, but it wasn't until computerized radiative transfer calculations could be performed (by Gilbert Plass in the 1950s) that the definite potential for surface warming from upper-atmosphere gases - even in the bands which H2O utterly saturates at the surface - could be established.
https://www.aip.org/history/climate/co2.htm
Maybe in another 70 years, Longview and Lord of Planar will finally get the memos.